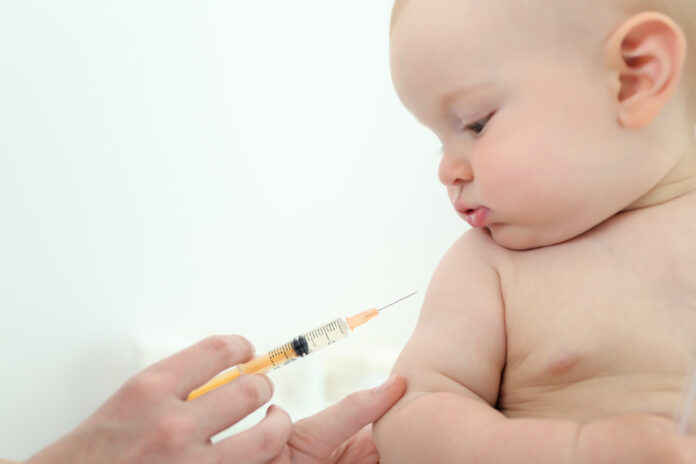
WASHINGTON: COVID-19 vaccinations for children under 5 hit another monthslong delay Friday as US regulators abruptly put the brakes on their efforts to speed review of the shots that Pfizer is testing for youngsters.
The US Food and Drug Administration, worried about the omicron variant’s toll on kids, had taken the extraordinary step of urging Pfizer to apply for OK of the extra-low dose vaccine before it’s clear if tots will need two shots or three. The agency’s plan could have allowed vaccinations to begin within weeks.
But Friday, the FDA reversed course and said it had become clear the agency needed to wait for data on how well a third shot works for the youngest age group. Pfizer said in a statement that it expected the data by early April.
FDA’s vaccine chief Dr. Peter Marks said he hoped parents would understand that the agency’s decision was part of its careful scientific review of the evidence Pfizer has submitted so far.
That information “made us realize that we needed to see data from a third dose from the ongoing trial in order to make a determination,” Marks told reporters. “We take our responsibility for reviewing these vaccines very seriously because we’re parents as well.”
The nation’s 18 million children under 5 make up the only age group not yet eligible for vaccination.
Rachel Perera, the mother of an 8-month-old from Los Angeles, said Friday’s news felt “like the rug just got pulled out from under me.”
After consulting with her pediatrician, Perera hoped a vaccine would be available this winter, or in early 2022 at the latest. The education policy researcher and her husband are caring for their child to avoid the unpredictability and risks of child care during a pandemic. But that means working on her dissertation for the Ph. D. she is pursuing when her child sleeps. On top of that, the daily calculations of risks, she says, have left her with “decision fatigue.”
“I’m just tired, and it feels like ‘when is this going to end’?” Perera said. “It feels like people around us are moving on with their lives, and we’re being left behind.”
Vaccine experts had been concerned with the sudden race to evaluate Pfizer’s vaccine — and now wonder what parents will make of the back-and-forth.
“I think they made the right decision to be careful and wait for the third-dose data,” said Dr. Jesse Goodman of Georgetown University, a former FDA vaccine chief.
“It was great to hear that there might be some promising data from two doses but it came out as ‘Hey everybody, you can expect a vaccine in a few weeks,'” he added. “I think this messaging gets very confusing for people.”
It’s not the first delay. Pfizer originally had expected to know by late December if the extra-low doses worked for kids under 5 — only to face a disappointing setback. Preliminary study results showed two shots were safe and strong enough to give good protection to babies as young as 6 months. But once tots reached the preschool age — the 2- to 4-year-olds — two doses weren’t protective enough, prompting the addition of a third to the study.
So it was a surprise when a few weeks ago, FDA urged Pfizer and its partner BioNTech to go ahead and apply. Next week, the agency’s independent scientific advisers were set to publicly debate if it was OK to start giving tots two shots before there was proof that a third would give them the extra needed protection — a highly unusual move.
Friday, the FDA abruptly canceled that meeting, promising to hold it once Pfizer submits the third-dose evidence. Even if Pfizer completes its submission by early April, it will take the FDA and other health authorities several weeks to review and publicly vet the data.
Earlier this week, FDA’s Marks had promised the agency wouldn’t cut corners but also noted how rapidly the pandemic was changing. Before Thanksgiving, no one had heard of omicron, by last month pediatric COVID-19 infections had hit an all-time high — and now cases are dropping fast as the latest mutant burns out.
How long to wait for new vaccine data — and how much to require — is a difficult balancing act for the FDA. It is caught between pressure to be more proactive against a rapidly changing virus and the risk that acting too quickly may deter families already on the fence about vaccinating their children.
Pfizer aims to give children as young as 6 months shots that contain one-tenth of the dose given to adults — two shots three weeks apart followed by a third at least two months later.
That’s a smaller dose than youngsters ages 5 to 11 receive, a third of the adult dose.
Vaccination rates have been lower among children than in other age groups. As of last week, just 22 percent of kids ages 5 to 11 and just over half of 12- to 17-year-olds were fully vaccinated, according to the American Academy of Pediatrics. Nearly three-quarters of adults are fully vaccinated.
A Kaiser Family Foundation poll taken last month found just 3 in 10 parents of children under 5 would get their youngster vaccinated as soon as shots were authorized, while about a quarter said they definitely would not.
Dr. Moira Szilagyi, the pediatricians group’s president, recognized parental frustration but said in a statement that doctors were committed to “a careful, robust and transparent process to evaluate the evidence.”
Dr. Natasha Burgert, a pediatrician in Overland Park, Kansas, said, “We’re just gutted. We need this protection for our kids.”
She said some families likely feel relieved “because they didn’t want to make that decision without good efficacy data. Other groups of parents are just crying out, ‘Give us a choice. Show us what you’ve got and let us make a choice. Let us have access to it.”’
Sign in
Welcome! Log into your account
Forgot your password? Get help
Password recovery
Recover your password
A password will be e-mailed to you.